Adding Products to the Portal
Overview
In order for a practice to start ordering or selling things we must first add products to Legrande’s Product Catalog and link them to the manufacturer that fulfills that product. In this guide we will go over the steps to create a new product and add that product to Legrande’s product Catalog.
Who Can Create New Products?
Users with the “Administrate Legrande System” permission can create and edit products in the software. By default the following user roles have that permission turned on:
- Admin
How to Create a New Product
1. Click “Product” > “Catalog”
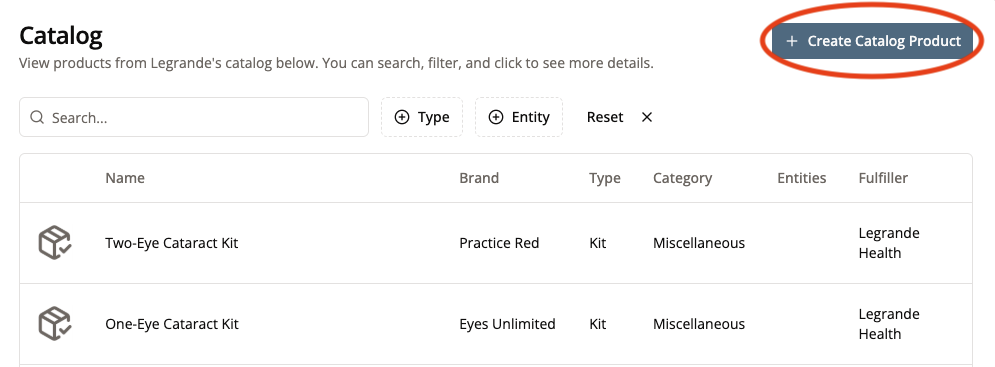
2. Click “Create Catalog Product”
3. Select the “Product Base” from the dropdown menu
Product Bases are the most generic definition of a product. For example, we could have multiple SKUs in the portal for ibuprofen. Each SKU could be manufactured by a different company or could have different pill sizes or quantities. At the end of the day though, they are all still ibuprofen and therefore share the same base: “Ibuprofen”.
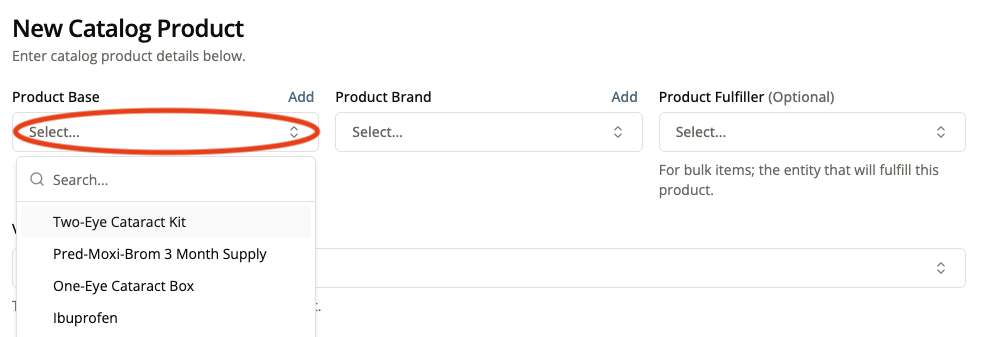
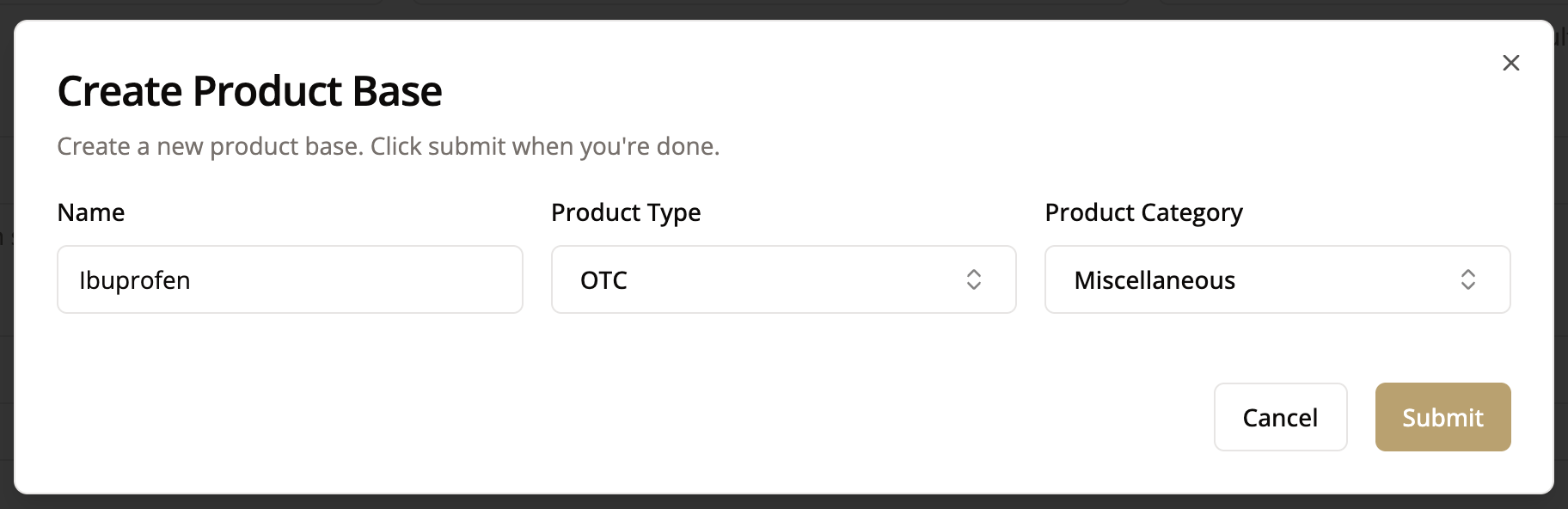
If your product base is not in the drop down menu, create a new one by clicking “Add”

DO
- Use generic names like Ibuprofen
DON’T
- List specific Brands like Advil
- Mention sizes
- Specify particular SKU numbers
- Specify strength, dosage, or administration route
Brand, size, SKU number, strength, dosage, and administration route will all be defined later
RX: a product that requires a doctor prescription to dispense
OTC: an Over The Counter product that doesn’t require a prescription
Kit: A bundle of products
This is a catch all section where users can specify a category for the product that’s useful for data analytics later. Some examples of categories are “glasses”, “eye drops”, “eye medication”, but really any category could be made and used.
Go to https://legrandehealth.app/product/categories/lookup to add new product categories.
4. Select a Product Brand from the dropdown menu (or add a new one by clicking “Add”)
Continuing with our example of adding Ibuprofen to the portal, we would specify a brand name like Equate (Walmart’s generic brand) or Advil.
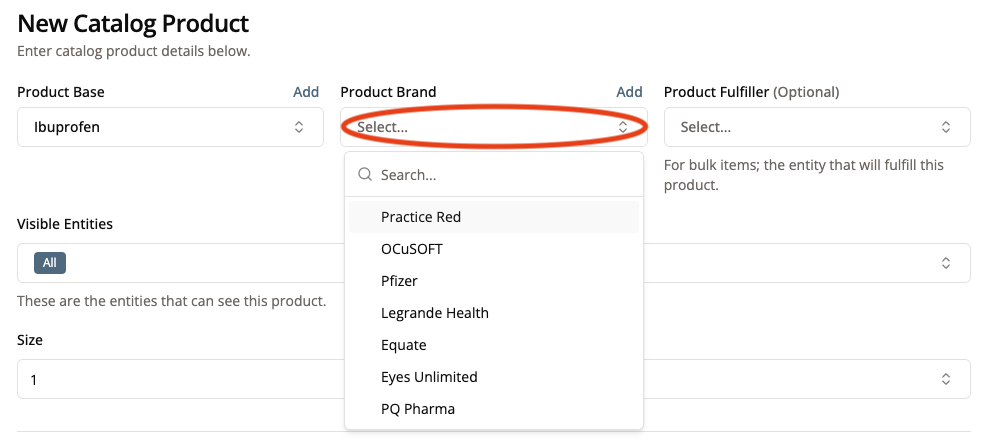
This is the brand name that appears on the product itself, not the name of the entity that actually fulfills the product.
For example, a cup with a practice’s branding on it would have the practice’s name as its brand even though the cup is actually fulfilled by Legrande.
For truly unbranded items like a blank canvas bag, use the brand “Unbranded”.
Generally though, strive to label things with their brand name if the brand is present on the product. For example, even though equate ibuprofen is a generic product, it still has the equate branding on the bottle. Therefore the brand for the product should be “Equate” not “Unbranded”.
5. Select a Product Fulfiller
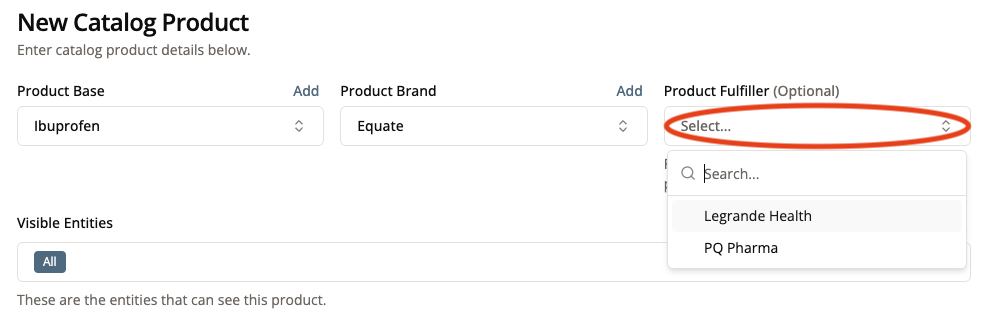
The product fulfiller is the entity that fulfills this product for bulk orders. Examples include Legrande Health, PQ Pharma, and Imprimis.
If this product is to be sold in Practice 360 (Bulk Ordering) then you must list the product’s fulfiller.
Product Fulfiller is optional for products to be sold in Patient 360.
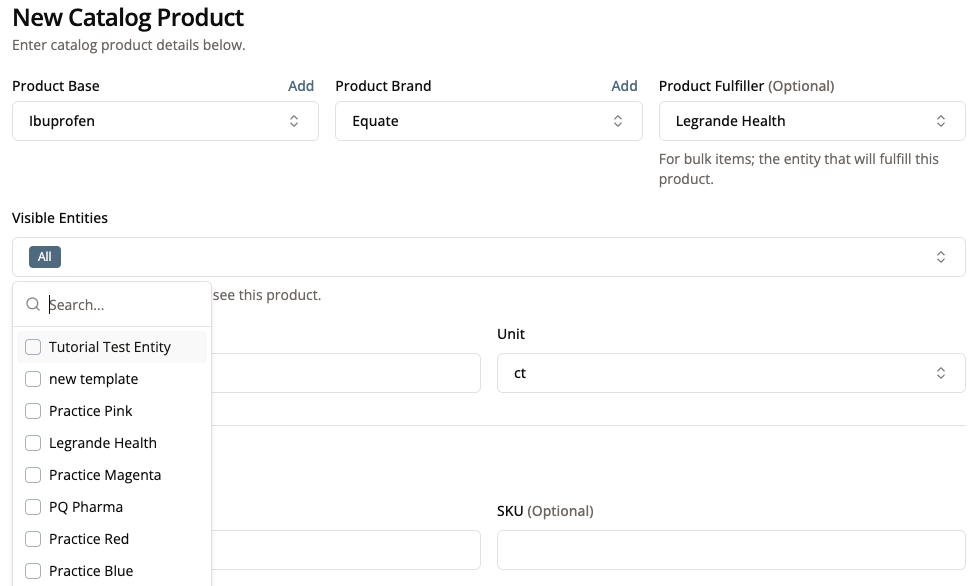
6. Select the Entities That Can See This Product in the Portal
Practices are allowed to add any products from the Product Catalog to their Practice Products for Sale. There are cases though where we might not want to allow a practice to order a specific product such as a kit designed for another practice. By selecting the Visible Entities for a product, only those entities can have the product added to their practice’s products for sale in Patient 360 or the list of products that the practice can purchase in Practice 360.
If no entities are selected, then all entities will be able to add this product to their products for sale or purchase.
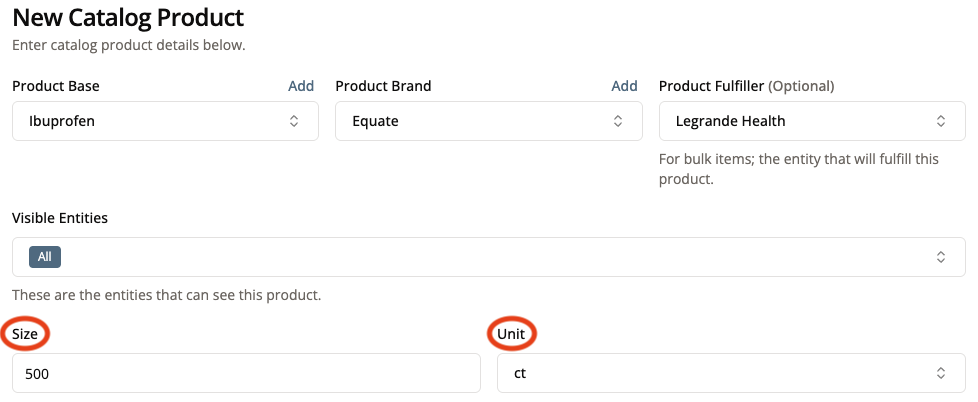
7. Specify the Size and Unit of Measurement
All products need a size specified. For an individual product like a single pair of glasses or an individual kit, the size should be 1 ct. The size should be the total size of the whole product not the size of a single dose of the product.
In the case of the ibuprofen bottle that we are adding the size is 500 ct. The size of an individual dose comes later in the Strength, Dosage, and Administration Route section.
8. Specify the NDC Code and SKU
NDC Code is an optional field for non-medication products like medical tape and eye shields.
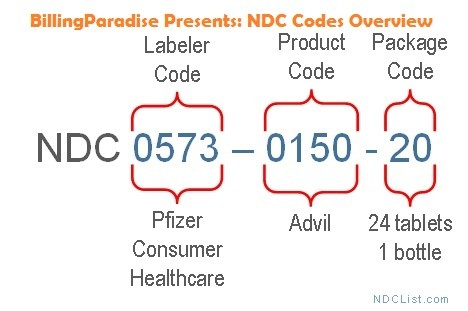
NDC stands for National Drug Code. It is a unique code assigned to every drug in the US that is monitored by the FDA.
The first 4 to 5 digits are the labeler code; the unique code assigned to the manufacturer of the drug.
The next 3 to 4 digits are the product code which identifies the unique product line this product belongs to.
The last 2 digits are the packaging code which identifies the unique size of the container of the product.
SKU stands for Stock Keeping Unit. It can be any string used internally by Legrande to uniquely identify this exact product. Two products with the same SKU should be identical in every way including the packaging and the product’s contents.
9. Specify Strength, Dosage, and Administration Route
Strength, Dosage, and Administration Route are optional fields because they don’t apply to non-medications like medical tape and glasses.
Strength refers to the concentration of the drug and can vary wildly based on the type of drug. This is a free text field and can range from a percent concentration of the active ingredient in a liquid drug to the size of drug capsules.
Dosage refers to the form that the drug comes in. The options are:
- Suspension - the drug is visibly separated from the liquid it rests in
- Emulsion - two liquids that often separate from each other mixed together
- Gel - a thin water based liquid
- Ointment - a thick oil based liquid
- Capsule - a dissolvable exterior that encases the medication
- Tablet - a solid pill
- Solution - the medication is fully dissolved and is indistinguishable from the other ingredients of the bottle
How the drug is to be taken. The options are
- Ophthalmic - applied directly to the eye
- Oral - ingested through the mouth
10. Fill Out Pricing Information
A pricing benchmark commonly used in the pharmacy industry to determine reimbursement rates for prescription medications. It represents an estimated cost of a drug based on reported wholesale prices but does not necessarily reflect the actual price paid by pharmacies. AWP is often higher than the true acquisition cost and is a reference point for insurance claim adjudication.
Example:
Good Day Pharmacy purchases Restasis for $500, but the AWP for Restasis is $600. When submitting a claim to the insurance company, Good Day uses the AWP-based pricing model and submits a claimed value of $600. The insurance reimburses them AWP minus a contractual discount (e.g., AWP - 5%), which results in a reimbursement of $575. If Good Day had instead submitted a claim for the actual acquisition cost of $500, they might only be reimbursed exactly $500, resulting in no margin to cover operational costs. Using AWP allows the pharmacy to account for pricing models negotiated with Pharmacy Benefit Managers (PBMs) and ensure sustainable reimbursement.
This serves no functional purpose in the portal yet, but will be used for analytics in the future.
The suggested price a practice should sell this product to patients for in Patient 360. The practice can still set their own price when they add the product to their Practice’s Products.
11. Add a Description and Upload a Product Image

12. Click “Next” to review what components your product contains.
Some examples of products that would not have other components:
Pred-Moxi-Brom
Eye Shields
Ibuprofen
Some examples of products that would need other components:
One-Eye Cataract Kit (Pred-Moxi-Brom, Artifical Tears, Eyelid Wipes)
Shield and Tape (Eye Shield, Paper Tape)
A multi-pack of a product (such as a 3-month supply of Pred-Moxi-Brom)
If your product is a single component, click “Skip” to complete the process of creating your new product.
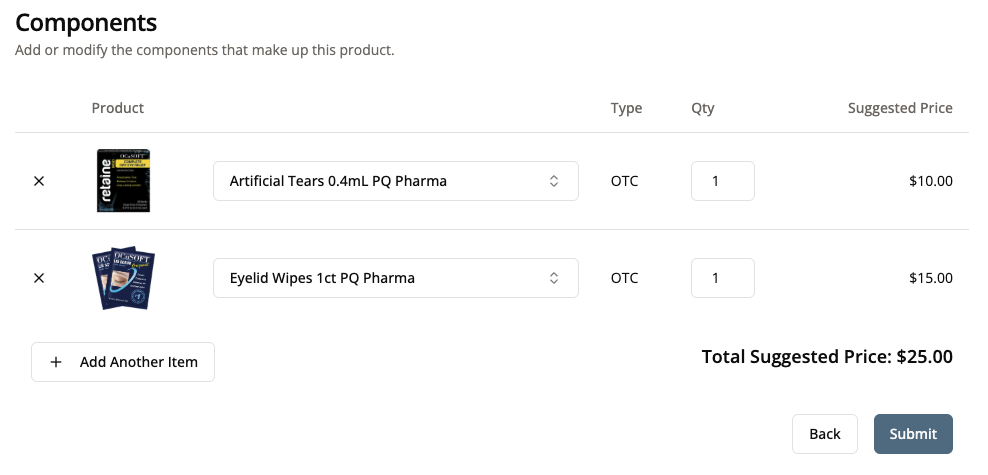
If your product is made up of more than one component, add the components your kit requires.
- For example, if you created a Dry Eye Supplement Kit as your product, you would need to add items (such as saline, eyelid wipes, or artificial tears) that are needed for your kit. Once you have added all necessary components, click “Submit.”
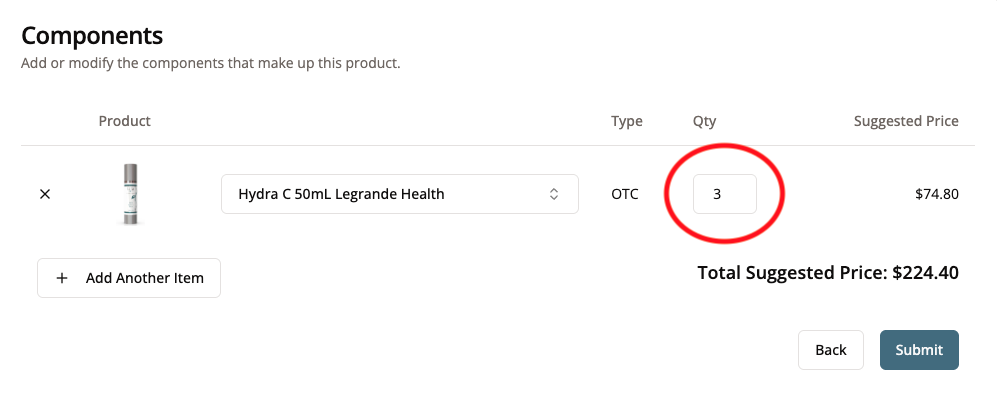
- If you were making a multi-pack of a product, you would need to make sure you add all components. For example, someone making a 3-month supply or multi-pack might say the multi-pack needs 3 of the bottles.
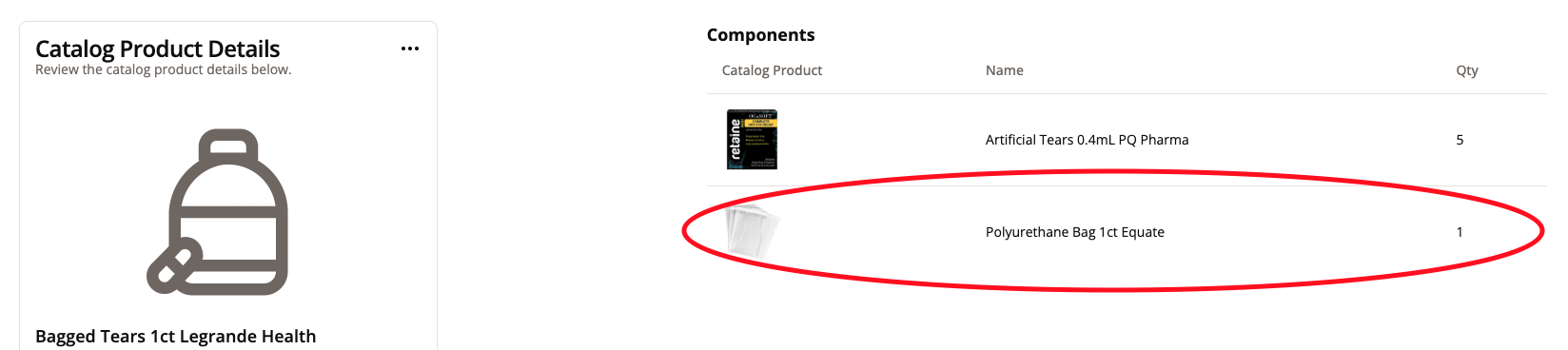
When adding components, make sure to add EVERY possible component the Warehouse will need to have enough of to assemble a product.
For example, if the team will need to combine the products in a bag or use tape for the product, make sure to include it with the components.
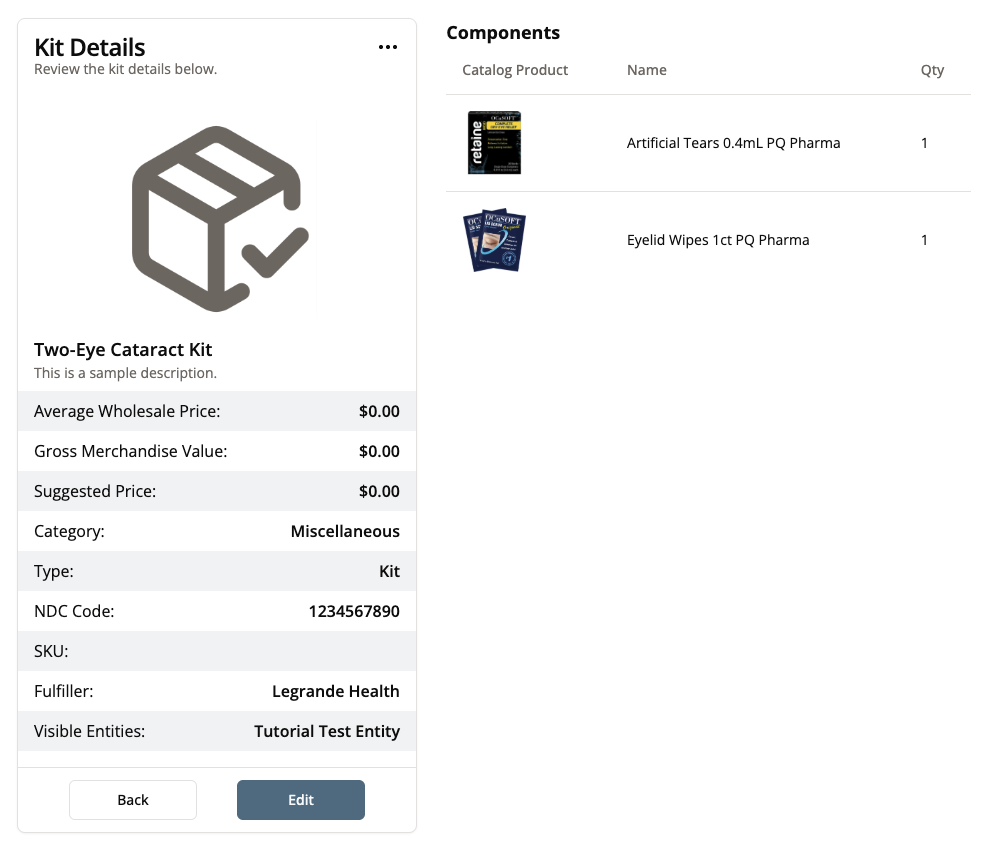
Once you have clicked “Submit,” you’ll be able to view and edit the product you just added.
Conclusion
If all went according to plan, you can now move on to adding this product to a practice’s products for sale in Patient 360 or you can add it as a product a practice can purchase in bulk from a manufacturer in Practice 360.
Next Steps
List a product for sale at a practice or dispenser for use in Patient 360:
List a product for purchase from a manufacturer in Practice 360: